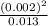Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

You know the right answer?
0.030 moles of a weak acid, HA, was dissolved in 2.0 L of water to form a solution. At equilibrium,...
Questions



Mathematics, 04.08.2019 03:30

Health, 04.08.2019 03:30








Computers and Technology, 04.08.2019 03:30

Computers and Technology, 04.08.2019 03:30

Computers and Technology, 04.08.2019 03:30

Computers and Technology, 04.08.2019 03:30

Computers and Technology, 04.08.2019 03:30

Computers and Technology, 04.08.2019 03:30



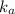 for the weak acid is
for the weak acid is 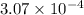 .
.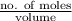
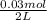
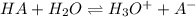
![k_{a} = \frac{[A^{-}][H_{3}O^{+}]}{[HA]}](/tpl/images/0571/0012/aea8e.png)
![\frac{x^{2}}{[HA]}](/tpl/images/0571/0012/57461.png)