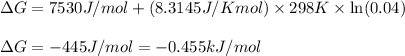Chemistry, 30.03.2020 21:38 zhuotingwu147
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate is the product. dihydroxyacetone phosphate − ⇀ ↽ − glyceraldehyde − 3 − phosphate the standard change in Gibbs free energy is Δ G ° ' = 7.53 kJ/mol . Calculate Δ G for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde-3-phosphate] = 0.00400 M . Δ G = kJ / mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
For the aqueous reaction dihydroxyacetone phosphate is the reactant and glyceraldehyde 3 phosphate i...
Questions

Chemistry, 22.03.2021 02:50



Business, 22.03.2021 02:50

English, 22.03.2021 02:50

Mathematics, 22.03.2021 02:50

Computers and Technology, 22.03.2021 02:50

Health, 22.03.2021 02:50

History, 22.03.2021 02:50


Mathematics, 22.03.2021 02:50


Mathematics, 22.03.2021 02:50




History, 22.03.2021 02:50



Mathematics, 22.03.2021 02:50

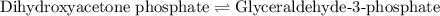
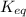 of above equation is:
of above equation is:![K_{eq}=\frac{\text{[Glyceraldehyde-3-phosphate]}}{\text{[Dihydroxyacetone phosphate]}}](/tpl/images/0571/3632/f265a.png)
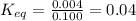
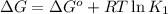
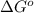 = Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol
= Standard Gibbs free energy = 7.53 kJ/mol = 7530 J/mol