
Chemistry, 30.03.2020 20:27 Lovelybunny321
Instead of using ratios for back titrations we can also use molarities, if our solutions are standardized. A 0.196 g sample of antacid containing an unknown amount of triprotic base Al(OH)3 was reacted with 25.0mL of 0.111M HCl. The resulting solution was then titrated with 11.05mL of 0.132M NaOH solution. Calculate the mass percent of Al(OH)3 in the antacid sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

You know the right answer?
Instead of using ratios for back titrations we can also use molarities, if our solutions are standar...
Questions


Mathematics, 13.05.2021 20:20

Health, 13.05.2021 20:20

Social Studies, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20



Mathematics, 13.05.2021 20:20

English, 13.05.2021 20:20





Mathematics, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20


Mathematics, 13.05.2021 20:20


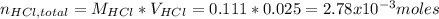
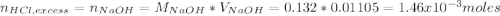
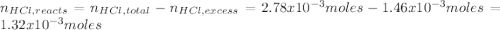
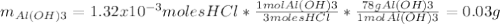
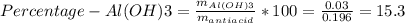 %
%

