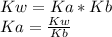Chemistry, 30.03.2020 20:27 viktoria1198zz
If you know Kb for ammonia, NH3, you can calculate the equilibrium constant, Ka, for the following reaction: NH4+ NH3 + H+ by the equation: Ka = 1 / Kb Ka = Kb / Kw Ka = Kw / Kb Ka = Kw × Kb None of these choices are correct.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
If you know Kb for ammonia, NH3, you can calculate the equilibrium constant, Ka, for the following r...
Questions

Advanced Placement (AP), 20.09.2020 04:01


Social Studies, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Biology, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01


History, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



English, 20.09.2020 04:01

History, 20.09.2020 04:01


Physics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01