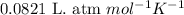Chemistry, 30.03.2020 20:35 esanchez2002fcb
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel at 23.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

You know the right answer?
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel...
Questions

Physics, 04.08.2019 20:30

Geography, 04.08.2019 20:30



History, 04.08.2019 20:30


History, 04.08.2019 20:30

History, 04.08.2019 20:30

Biology, 04.08.2019 20:30

History, 04.08.2019 20:30

Business, 04.08.2019 20:30

History, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30

Chemistry, 04.08.2019 20:30

History, 04.08.2019 20:30


Mathematics, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30

History, 04.08.2019 20:30


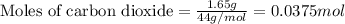
![23^oC=[23+273]=296K](/tpl/images/0571/0457/eb892.png)