

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Assuming no change in temperature and pressure, calculate the volume of O2 (in liters) required for...
Questions

Mathematics, 17.11.2020 18:40

Mathematics, 17.11.2020 18:40


Mathematics, 17.11.2020 18:40

Mathematics, 17.11.2020 18:40

Health, 17.11.2020 18:40

Business, 17.11.2020 18:40

History, 17.11.2020 18:40

Mathematics, 17.11.2020 18:40


History, 17.11.2020 18:40



History, 17.11.2020 18:40




Mathematics, 17.11.2020 18:40


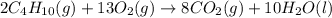
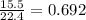 mole of butane gas
mole of butane gas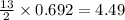 moles of oxygen gas
moles of oxygen gas

