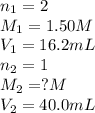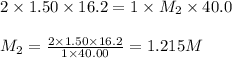Chemistry, 30.03.2020 21:17 nannagarvey9945
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard solution of sulfuric acid (H2SO4H2SO4). What was the molarity of the KOHKOH solution if 16.2 mLmL of 1.50 MM H2SO4H2SO4 was needed? The equation is 2KOH(aq)+H2SO4(aq)→K2SO4(aq)+2H2O(l )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard soluti...
Questions



Arts, 19.01.2021 20:40


Mathematics, 19.01.2021 20:40


Biology, 19.01.2021 20:40


Mathematics, 19.01.2021 20:40

Mathematics, 19.01.2021 20:40

Mathematics, 19.01.2021 20:40

Chemistry, 19.01.2021 20:40

Mathematics, 19.01.2021 20:40



Mathematics, 19.01.2021 20:40

English, 19.01.2021 20:40



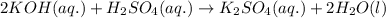
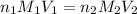
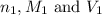 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 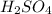
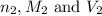 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.