
Chemistry, 30.03.2020 20:46 joannegrace869
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of acetylene needed to produce 2.00 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat pr...
Questions



Computers and Technology, 10.10.2019 17:20










Physics, 10.10.2019 17:20

Computers and Technology, 10.10.2019 17:20

Physics, 10.10.2019 17:20





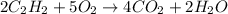
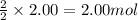 of acetylene gas
of acetylene gas

