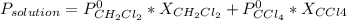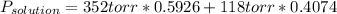Chemistry, 30.03.2020 21:07 villarrealc1987
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) among the many cancer causing volatile chlorinated organic compounds. An autoshop you are employed at keeps a jar (closed) of this solution to use as a solvent for cleaning parts. If the jar contains 1.60mol of dichloromethane and 1.10mol of CCl4, what would be the total pressure in the jar if the shop is kept at a consistent 23.5C

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) a...
Questions

Spanish, 30.10.2020 18:40

Chemistry, 30.10.2020 18:40


Mathematics, 30.10.2020 18:40




Social Studies, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Computers and Technology, 30.10.2020 18:40

Chemistry, 30.10.2020 18:40



History, 30.10.2020 18:40

Business, 30.10.2020 18:40

Business, 30.10.2020 18:40



Business, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40