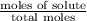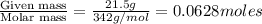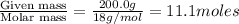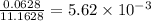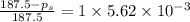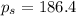Chemistry, 30.03.2020 21:00 shelatzcreed
Calculate the vapor pressure of water above a solution prepared by adding 21.5 g of lactose (C12H22O11) to 200.0 g of water at 338 K. (Vapor-pressure of water at 338 K 187.5 torr.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Calculate the vapor pressure of water above a solution prepared by adding 21.5 g of lactose (C12H22O...
Questions

Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00



Biology, 03.03.2021 01:00

English, 03.03.2021 01:00

English, 03.03.2021 01:00

English, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Computers and Technology, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Social Studies, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00


Chemistry, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

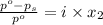
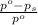 = relative lowering in vapor pressure
= relative lowering in vapor pressure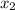 = mole fraction of solute =
= mole fraction of solute =