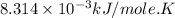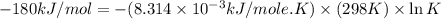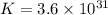Chemistry, 30.03.2020 21:03 manyah6189
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(s) + 2Ag+ (aq) LaTeX: \longrightarrow⟶ Pb2+(aq) + 2Ag(s) As a consequence, silver is a valuable byproduct in the industrial extraction of lead from its ores. Calculate K and LaTeX: \DeltaΔG° at 298K for this reaction. E°cell = .93 K: Enter as e notation to 1 decimal place (eg 1.2e3) LaTeX: \DeltaΔG°: Enter in kJ/mol to 0 decimal places. Use 96.5 kJ/Vmol e- for F (Faraday's constant). Do not use e notation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(...
Questions




Computers and Technology, 01.10.2019 15:30

English, 01.10.2019 15:30

Social Studies, 01.10.2019 15:30

Mathematics, 01.10.2019 15:30



Mathematics, 01.10.2019 15:30


History, 01.10.2019 15:30

Mathematics, 01.10.2019 15:30

History, 01.10.2019 15:30



Arts, 01.10.2019 15:30



Social Studies, 01.10.2019 15:30

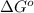 and K is, -180 kJ/mol and
and K is, -180 kJ/mol and 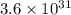
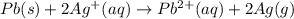
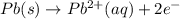
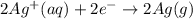
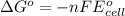
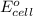 = standard electrode potential of the cell = 0.93 V
= standard electrode potential of the cell = 0.93 V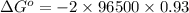
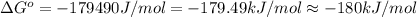
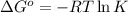
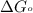 = standard Gibbs free energy = -180 kJ/mol
= standard Gibbs free energy = -180 kJ/mol