
Chemistry, 30.03.2020 21:01 gungamer720
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in the reaction of bromine and chlorine to form BrCl, if 29.7 g of Br2 and 11.2 g of Cl2 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in t...
Questions

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

English, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01

Biology, 21.10.2020 20:01

History, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

English, 21.10.2020 20:01

Chemistry, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

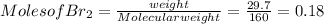
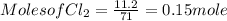
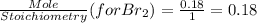
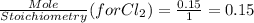
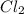 is a limiting reactant and Br₂ is excess reactant.
is a limiting reactant and Br₂ is excess reactant.

