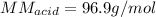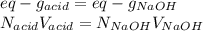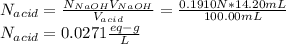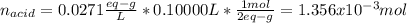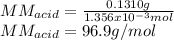Chemistry, 30.03.2020 21:27 saadizak7098
6. A 0.1310 g sample of an unknown diprotic acid is diluted to 100.00 mL and titrated by using 0.1910 M NaOH. If 14.20 mL of the NaOH solution is required to reach the second equivalence point, what is the molar mass of the acid?

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
6. A 0.1310 g sample of an unknown diprotic acid is diluted to 100.00 mL and titrated by using 0.191...
Questions

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00


Mathematics, 26.04.2021 22:00

English, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00


English, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00






Chemistry, 26.04.2021 22:00


History, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00