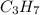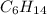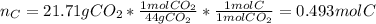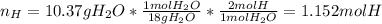Chemistry, 30.03.2020 21:23 jaleelbrown80
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams of CO2 and 10.37 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 86.18 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon.

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams...
Questions

Chemistry, 22.06.2019 05:30


World Languages, 22.06.2019 05:30




Physics, 22.06.2019 05:30









Mathematics, 22.06.2019 05:30


Physics, 22.06.2019 05:30

English, 22.06.2019 05:30

Chemistry, 22.06.2019 05:30