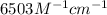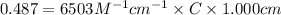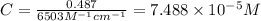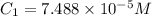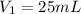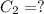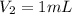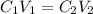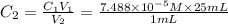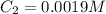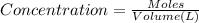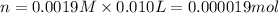An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 327 nm is 0.487 in a 1.000 cm cuvette. The molar absorptivity for this compound at 327 nm is ϵ 327 = 6503 M^(−1) cm^(−1).
(a) What is the concentration of the compound in the cuvette?
(b) What is the concentration of the compound in the 10-mL flask?
(c) How many milligrams of the compound were used to make the 10-mL solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volume...
Questions

Mathematics, 18.03.2021 02:40


English, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40



History, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Social Studies, 18.03.2021 02:40


History, 18.03.2021 02:40

History, 18.03.2021 02:40

Health, 18.03.2021 02:40

History, 18.03.2021 02:40

History, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40

English, 18.03.2021 02:40

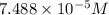 is the concentration of the compound in the cuvette.
is the concentration of the compound in the cuvette.
 = molar absorptivity of this solution =
= molar absorptivity of this solution =