
Chemistry, 30.03.2020 22:35 tyrareed702
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many liters of sulfur dioxide, measured at STP, are produced by the reaction of 3.73 kg of galena with 170. L of oxygen gas at 220°C and 2.00 atm? Lead(II) oxide also forms.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many li...
Questions









Computers and Technology, 12.11.2019 03:31









Computers and Technology, 12.11.2019 03:31


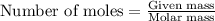
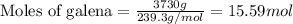
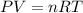
![220^oC=[220+273]K=493K](/tpl/images/0571/5966/b1a8e.png)
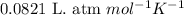
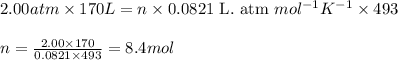
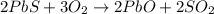
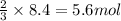 of galena
of galena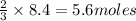 of sulfur dioxide
of sulfur dioxide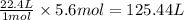 of volume
of volume

