Chemistry, 30.03.2020 22:32 savannahvargas512
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O. Calculate the maximum mass (in grams) of selenic acid, H2SeO4, that can be produced in the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

You know the right answer?
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O....
Questions

Mathematics, 08.04.2020 22:24

History, 08.04.2020 22:24


Mathematics, 08.04.2020 22:24

Mathematics, 08.04.2020 22:24


Mathematics, 08.04.2020 22:24


Physics, 08.04.2020 22:24

Social Studies, 08.04.2020 22:24



English, 08.04.2020 22:24



Mathematics, 08.04.2020 22:24




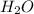
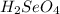 produced is, 220.4 grams.
produced is, 220.4 grams.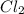 = 431 g
= 431 g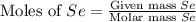
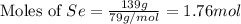
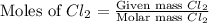
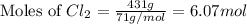
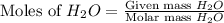
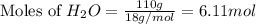
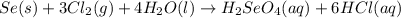
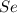
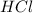 react with
react with 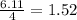 moles of
moles of 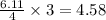 moles of
moles of 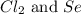 are excess reagent because the given moles are greater than the required moles and
are excess reagent because the given moles are greater than the required moles and