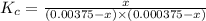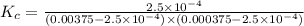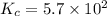3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark with HNO3 in 10 mL volumetric flask. The blood-red [Fe(NCS)]2+ ion that forms has an equilibrium molar concentration of 2.5*10-4 mol/L as determined from the calibration plot. Calculate the Kc for [Fe(NCS)]2+ formation. Assume the volumes are additive.

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

You know the right answer?
3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark wit...
Questions


Physics, 25.12.2020 16:00



Mathematics, 25.12.2020 16:00

Chemistry, 25.12.2020 16:00














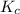 for
for ![[Fe(NCS)]^{2+}](/tpl/images/0571/7802/c2420.png) formation is
formation is  .
.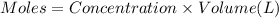
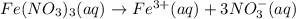
![[Fe(NO_3)_3]=0.02 M=[Fe^{3+}]](/tpl/images/0571/7802/da55e.png)
![[Fe^{3+}]=0.02 M](/tpl/images/0571/7802/a8178.png)
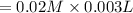
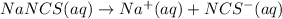
![[NaNCS]=0.002 M=[NCS^-]](/tpl/images/0571/7802/ed7be.png)
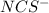 ion =
ion = ![[NCS^{-}]=0.002 M](/tpl/images/0571/7802/84149.png)
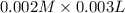
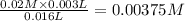
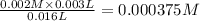
![Fe^{3+}+NCS^-\rightleftharpoons [Fe(NCS)]^{2+}](/tpl/images/0571/7802/1aeb7.png)
![[Fe(NCS)]^{2+}=x=2.5\times 10^{-4} M](/tpl/images/0571/7802/1a7a9.png)
![K_c=\frac{[[Fe(NCS)]^{2+}]}{[Fe^{3+}][NCS^-]}](/tpl/images/0571/7802/4616c.png)