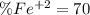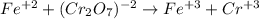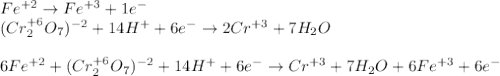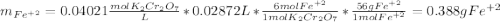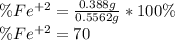Chemistry, 30.03.2020 23:08 GreenHerbz206
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solution. This solution is then titrated with 28.72 mL of 0.04021 M K2Cr2O7 (aq). What is the percent by mass iron in the ore sample

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solut...
Questions






Business, 21.04.2021 17:50


Mathematics, 21.04.2021 17:50


Health, 21.04.2021 17:50

Social Studies, 21.04.2021 17:50




Mathematics, 21.04.2021 17:50


Mathematics, 21.04.2021 17:50

English, 21.04.2021 17:50


English, 21.04.2021 17:50