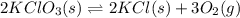Chemistry, 30.03.2020 22:56 prince2195
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. a. CO3 2-(aq) + H2O(I)HCO3 -(aq) + OH-(aq) b. 2 KCIO3(s)2 KCI(s) + 3 O2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
You know the right answer?
Write an equilibrium expression for each chemical equation involving one or more solid or liquid rea...
Questions


Mathematics, 29.08.2019 18:00

Mathematics, 29.08.2019 18:00

Mathematics, 29.08.2019 18:00




Chemistry, 29.08.2019 18:00





Physics, 29.08.2019 18:00




Social Studies, 29.08.2019 18:00

Mathematics, 29.08.2019 18:00


![K=\frac{[HCO_3^-][OH^-]}{[CO_3^{2-}]}](/tpl/images/0571/6769/f9db2.png)
![K=[O_2]^3](/tpl/images/0571/6769/a8fdd.png)
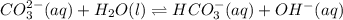
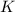 will be,
will be,