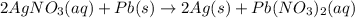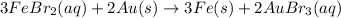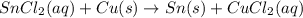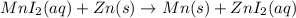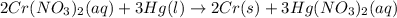Chemistry, 30.03.2020 23:13 jadajordan1010
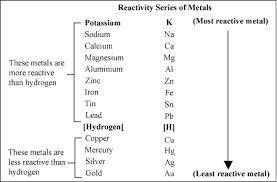
Based on the activity series, which one of the reactions below will occur? Based on the activity series, which one of the reactions below will occur? 3FeBr2 (aq) + 2Au (s) → 3Fe (s) + 2AuBr3 (aq) SnCl2 (aq) + Cu (s) → Sn (s) + CuCl2 (aq) Zn (s) + MnI2 (aq) → ZnI2 (aq) + Mn (s) 2AgNO3 (aq) + Pb (s) → 2Ag (s) + Pb(NO3)2 (aq) 3Hg (l) + 2Cr(NO3)3 (aq) → 3Hg(NO3)2 + 2Cr (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Based on the activity series, which one of the reactions below will occur? Based on the activity ser...
Questions