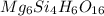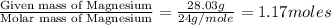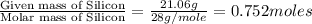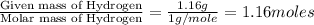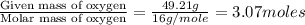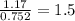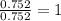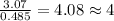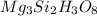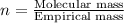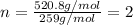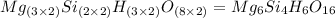Chemistry, 30.03.2020 23:25 genyjoannerubiera
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

You know the right answer?
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following...
Questions


Mathematics, 16.09.2021 01:20

Mathematics, 16.09.2021 01:20

English, 16.09.2021 01:20






Health, 16.09.2021 01:20



Mathematics, 16.09.2021 01:20



English, 16.09.2021 01:20



History, 16.09.2021 01:20

Mathematics, 16.09.2021 01:20

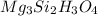 and
and