
Chemistry, 30.03.2020 23:33 mathman783
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? 4.10 × 106 s-1 713 s-1 1.36 × 102 s-1 2.65 × 104 s-1 3.69 × 104 s-1

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magni...
Questions










Chemistry, 14.05.2021 18:50

Spanish, 14.05.2021 18:50

History, 14.05.2021 18:50

Geography, 14.05.2021 18:50

Chemistry, 14.05.2021 18:50

Mathematics, 14.05.2021 18:50

Health, 14.05.2021 18:50


History, 14.05.2021 18:50


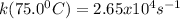
![\frac{k(75.0^0C)}{k(25.0^0C)} =exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]](/tpl/images/0571/8653/57013.png)
![k(75.0^0C)=k(25.0^0C)exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]\\\\k(75.0^0C)=1.35x10^2s^{-1}exp[-\frac{91000J/mol}{8.314J/(mol*K)}(\frac{1}{348.15K}-\frac{1}{298.15K} )]\\\\k(75.0^0C)=2.65 x 10^4 s^{-1}](/tpl/images/0571/8653/debb3.png)


