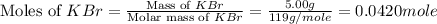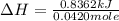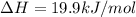Chemistry, 30.03.2020 23:33 PlzNoToxicBan
When a 5.00 g 5.00 g sample of KBr KBr is dissolved in water in a calorimeter that has a total heat capacity of 2.26 kJ ⋅ K − 1 , 2.26 kJ⋅K−1, the temperature decreases by 0.370 K . 0.370 K. Calculate the molar heat of solution of KBr .

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
When a 5.00 g 5.00 g sample of KBr KBr is dissolved in water in a calorimeter that has a total heat...
Questions






Advanced Placement (AP), 20.09.2020 18:01

Mathematics, 20.09.2020 18:01





Mathematics, 20.09.2020 18:01


Computers and Technology, 20.09.2020 18:01


Mathematics, 20.09.2020 18:01




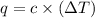
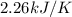
 = change in temperature = 0.370 K
= change in temperature = 0.370 K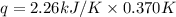
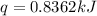
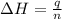
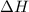 = enthalpy change = ?
= enthalpy change = ?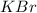 = 5.00 g
= 5.00 g