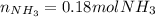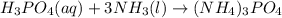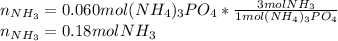Chemistry, 31.03.2020 00:07 caliharris123
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of ammonia needed to produce 0.060 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting...
Questions

Advanced Placement (AP), 24.12.2020 09:30

Mathematics, 24.12.2020 09:30

Biology, 24.12.2020 09:30


Social Studies, 24.12.2020 09:30

English, 24.12.2020 09:30

Health, 24.12.2020 09:30

English, 24.12.2020 09:30


Biology, 24.12.2020 09:30



Spanish, 24.12.2020 09:40


Business, 24.12.2020 09:40

Biology, 24.12.2020 09:40

History, 24.12.2020 09:40



Mathematics, 24.12.2020 09:40