Chemistry, 30.03.2020 23:46 abieber4328
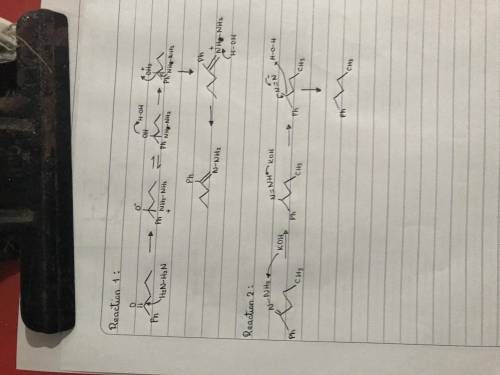
The Wolff-Kishner reaction involves the reaction of an aldehyde/ketone with hydrazine in the presence of KOH. The process is useful for converting an aldehyde or ketone into an alkane. The reaction involves formation of a hydrazone, followed by base-catalyzed double-bond migration, loss of N2 gas to give a carbanion, and protonation to give the alkane. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

You know the right answer?
The Wolff-Kishner reaction involves the reaction of an aldehyde/ketone with hydrazine in the presenc...
Questions

Mathematics, 12.05.2021 21:40




French, 12.05.2021 21:40

History, 12.05.2021 21:40


Computers and Technology, 12.05.2021 21:40


Medicine, 12.05.2021 21:40


Computers and Technology, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Social Studies, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Biology, 12.05.2021 21:40