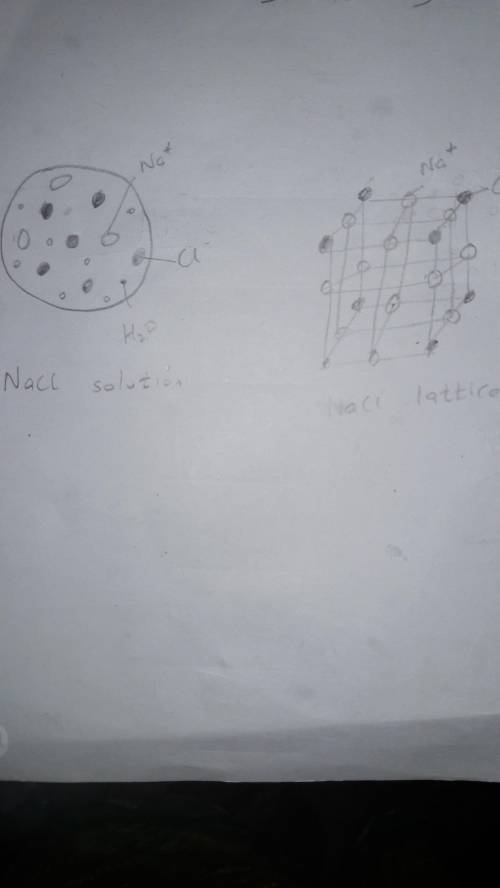
Classify the following as either a heterogeneous or homogeneous mixture, and explain your answers. a. orange juice b. tap water 2. a. What are substances called whose water solutions conduct electricity? b. Why does a salt solution conduct electricity? c. Why does a sugarwater solution not conduct electricity? 3. Make a drawing of the particles in an NaCl solution to show why this solution conducts electricity. Make a drawing of the particles in an NaCl crystal to show why pure salt does not conduct. 4. Describe one way to prove that a mixture of sugar and water is a solution and that a mixture of sand and water is not a solution. 5. Name the solute and solvent in the following: a. 14-karat gold b. corn syrup c. carbonated, or sparkling, water Critical Thinking 6. ANALYZING INFORMATION If you allow a container of sea water to sit in the sun, the liquid level gets lower and lower, and

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Classify the following as either a heterogeneous or homogeneous mixture, and explain your answers. a...
Questions

Mathematics, 02.06.2020 10:58


English, 02.06.2020 10:58





Computers and Technology, 02.06.2020 10:58


Mathematics, 02.06.2020 10:58

Mathematics, 02.06.2020 10:58


English, 02.06.2020 11:57

Mathematics, 02.06.2020 11:57


Mathematics, 02.06.2020 11:57

History, 02.06.2020 11:57

Mathematics, 02.06.2020 11:57

Mathematics, 02.06.2020 11:57