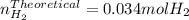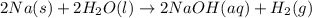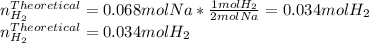Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
When sodium metal is added to water, the following reaction occurs: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H...
Questions

Physics, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20

Biology, 23.09.2019 23:20

Biology, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20




History, 23.09.2019 23:20

Chemistry, 23.09.2019 23:20


History, 23.09.2019 23:20


Computers and Technology, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20




Computers and Technology, 23.09.2019 23:20