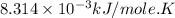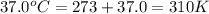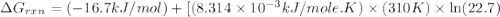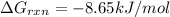Chemistry, 31.03.2020 00:01 samueltaye
Calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 22.7 and the temperature is 37.0 ° C ? Δ G ° ' for the reaction is − 16.7 kJ/mol .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2


Chemistry, 23.06.2019 11:00
What are the other two pieces of glassware you used in this experiment that you could obtain hundredths digit accuracy?
Answers: 2
You know the right answer?
Calculate the free energy change if the ratio of the concentrations of the products to the concentra...
Questions




Social Studies, 15.01.2020 20:31

English, 15.01.2020 20:31



English, 15.01.2020 20:31


Spanish, 15.01.2020 20:31

Chemistry, 15.01.2020 20:31

History, 15.01.2020 20:31

History, 15.01.2020 20:31


Spanish, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31


Mathematics, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31

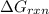 is -8.65 kJ/mol
is -8.65 kJ/mol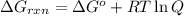 ............(1)
............(1)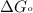 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol