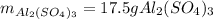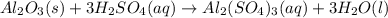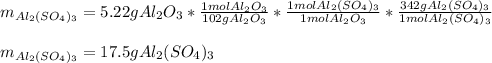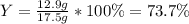Chemistry, 30.03.2020 23:55 guadalupemarlene2001
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The reaction yields 12.9 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) What is the theoretical yield of aluminum sulfate ?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The re...
Questions

Mathematics, 26.11.2019 23:31

History, 26.11.2019 23:31

Social Studies, 26.11.2019 23:31


History, 26.11.2019 23:31



Chemistry, 26.11.2019 23:31


Mathematics, 26.11.2019 23:31






Mathematics, 26.11.2019 23:31

Mathematics, 26.11.2019 23:31