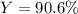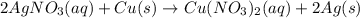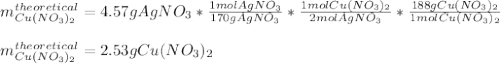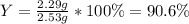For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yields 2.29 gram of copper(II) nitrate What is the percent yield for this reaction?Formula: % yield = (Actual yield/theoretical yield) x 100 2 AgNO3(aq) + Cu(s) à Cu(NO3)2 (aq) + 2 Ag(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1



Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yiel...
Questions

Mathematics, 09.09.2021 05:50


History, 09.09.2021 05:50

Biology, 09.09.2021 05:50


Mathematics, 09.09.2021 05:50

Mathematics, 09.09.2021 05:50

Mathematics, 09.09.2021 05:50

Chemistry, 09.09.2021 05:50


Mathematics, 09.09.2021 05:50



Mathematics, 09.09.2021 05:50


Mathematics, 09.09.2021 05:50

Mathematics, 09.09.2021 05:50

History, 09.09.2021 05:50

Mathematics, 09.09.2021 05:50