
Chemistry, 30.03.2020 23:54 dontcareanyonemo
Which change to an equilibrium mixture of this reaction results in the formation of more H2S? Which change to an equilibrium mixture of this reaction results in the formation of more ? a decrease in the volume of the reaction vessel (at constant temperature) an increase in temperature an increase in the amount of NH4HS in the reaction vessel all of the above

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
Which change to an equilibrium mixture of this reaction results in the formation of more H2S? Which...
Questions


Chemistry, 29.10.2020 23:50

Mathematics, 29.10.2020 23:50

Advanced Placement (AP), 29.10.2020 23:50

Mathematics, 29.10.2020 23:50

Spanish, 29.10.2020 23:50




Mathematics, 29.10.2020 23:50

Spanish, 29.10.2020 23:50

Biology, 29.10.2020 23:50





Biology, 29.10.2020 23:50



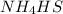 is endothermic:
is endothermic:
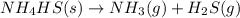
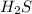 ?
?


