
Chemistry, 31.03.2020 00:13 electrofy456
The solid XY decomposes into gaseous X and Y: XY(s) ⇌ X(g) + Y(g) Kc = 4.1(at 0C) If the reaction is carried out in a 1-L container, which initial amounts of X and Y result in the formation of solid XY?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
The solid XY decomposes into gaseous X and Y: XY(s) ⇌ X(g) + Y(g) Kc = 4.1(at 0C) If the reaction is...
Questions

English, 02.02.2021 22:00

English, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00


Mathematics, 02.02.2021 22:00


History, 02.02.2021 22:00

Social Studies, 02.02.2021 22:00


English, 02.02.2021 22:00







Mathematics, 02.02.2021 22:00


English, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

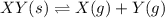 Kp = 4.1 (at 0°C)
Kp = 4.1 (at 0°C)![Q=[X][Y]](/tpl/images/0572/0334/b3caf.png)
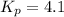
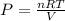
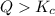 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.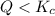 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.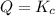 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

