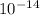Chemistry, 31.03.2020 00:18 NetherisIsTheQueen
At 100 degrees Celsius, the ion product for pure water is Kw= 51.3*10^-14. What is the pOH of water at this temperature? A) 7.00 B) 7.27 C) 6.14 D) 6.63 E) 7.86

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
At 100 degrees Celsius, the ion product for pure water is Kw= 51.3*10^-14. What is the pOH of water...
Questions

Mathematics, 16.04.2021 16:30



Mathematics, 16.04.2021 16:30


Mathematics, 16.04.2021 16:30







Mathematics, 16.04.2021 16:30


Health, 16.04.2021 16:30

Arts, 16.04.2021 16:30




History, 16.04.2021 16:30