Chemistry, 31.03.2020 00:27 shaymabejja1965
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solution of 2.00 M copper (II) chloride is available. How much of the stock solution is needed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solut...
Questions


History, 29.07.2019 23:30



History, 29.07.2019 23:30

Social Studies, 29.07.2019 23:30


History, 29.07.2019 23:30


Geography, 29.07.2019 23:30


History, 29.07.2019 23:30

Mathematics, 29.07.2019 23:30


History, 29.07.2019 23:30




History, 29.07.2019 23:30

Mathematics, 29.07.2019 23:30


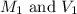 are the initial molarity and volume of copper (II) chloride.
are the initial molarity and volume of copper (II) chloride.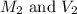 are the final molarity and volume of stock solution of copper (II) chloride.
are the final molarity and volume of stock solution of copper (II) chloride.