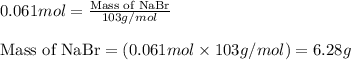Chemistry, 31.03.2020 00:27 coltonwsmith
Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . Suppose 17. g of hydrobromic acid is mixed with 2.44 g of sodium hydroxide. Calculate the maximum mass of sodium bromide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide an...
Questions




Mathematics, 29.01.2020 20:56


Chemistry, 29.01.2020 20:56


English, 29.01.2020 20:56


History, 29.01.2020 20:56

Mathematics, 29.01.2020 20:56



Mathematics, 29.01.2020 20:56

Business, 29.01.2020 20:56


Biology, 29.01.2020 20:56

Business, 29.01.2020 20:56

Biology, 29.01.2020 20:56

Biology, 29.01.2020 20:56

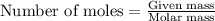 .....(1)
.....(1)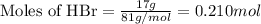
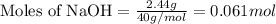

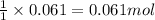 of HBr
of HBr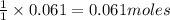 of carbon dioxide
of carbon dioxide