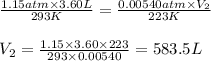A gas-filled balloon having a volume of 3.60 L at 1.15 atm and 20°C is allowed to rise to the stratosphere (about 30 km above the surface of the Earth), where the temperature and pressure are −50°C and 5.40 × 10−3 atm, respectively. Calculate the final volume of the balloon.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
A gas-filled balloon having a volume of 3.60 L at 1.15 atm and 20°C is allowed to rise to the strato...
Questions

Chemistry, 14.04.2021 04:40

Health, 14.04.2021 04:40







Biology, 14.04.2021 04:40

Mathematics, 14.04.2021 04:40

English, 14.04.2021 04:40

Social Studies, 14.04.2021 04:40

Mathematics, 14.04.2021 04:40

Mathematics, 14.04.2021 04:40

Mathematics, 14.04.2021 04:40


History, 14.04.2021 04:40


Mathematics, 14.04.2021 04:40

Mathematics, 14.04.2021 04:40

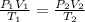
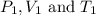 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas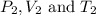 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas![P_1=1.15atm\\V_1=3.60L\\T_1=20^oC=[20+273]K=293K\\P_2=5.40\times 10^{-3}atm=0.00540atm\\V_2=?\\T_2=-50^oC=[-50+273]K=223K](/tpl/images/0572/1398/77ebd.png)