
Chemistry, 31.03.2020 00:47 KindaSmartPersonn
A chemist prepares a solution of ironIII chloride FeCl3 by measuring out 72.7mg of FeCl3 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl− anions in the chemist's solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
A chemist prepares a solution of ironIII chloride FeCl3 by measuring out 72.7mg of FeCl3 into a 100....
Questions




Mathematics, 23.01.2020 15:31

Mathematics, 23.01.2020 15:31



Mathematics, 23.01.2020 15:31


English, 23.01.2020 15:31





History, 23.01.2020 15:31


Arts, 23.01.2020 15:31


Physics, 23.01.2020 15:31

History, 23.01.2020 15:31

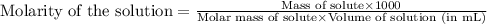
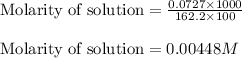
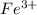 ions and 3 moles of
ions and 3 moles of 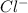 ions
ions

