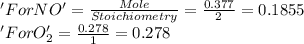Chemistry, 31.03.2020 00:46 kateferguson9852
Nitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.377 moles of nitrogen monoxide are mixed with 0.278 moles of oxygen gas. What is the formula for the limiting reagent? What is the maximum amount of nitrogen dioxide that can be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Nitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.37...
Questions

SAT, 05.11.2020 22:00

History, 05.11.2020 22:00

Social Studies, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00




Mathematics, 05.11.2020 22:00

World Languages, 05.11.2020 22:00


English, 05.11.2020 22:00


Social Studies, 05.11.2020 22:00

Geography, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00




Chemistry, 05.11.2020 22:00