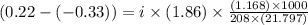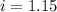Chemistry, 31.03.2020 01:05 raishagibson
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following the procedure in the lab manual, you determined that freezing point of water is 0.02oC. You weighed out 1.168 g of salt and added it to the original test tube, then determined that the freezing point was -0.33oC. Based on these experimental parameters, calculate the van't Hoff factor for BaCl2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following...
Questions

Business, 05.08.2019 00:00



Mathematics, 05.08.2019 00:00




Computers and Technology, 05.08.2019 00:00

Mathematics, 05.08.2019 00:00

Physics, 05.08.2019 00:00

Physics, 05.08.2019 00:00

Arts, 05.08.2019 00:00


Mathematics, 05.08.2019 00:00



History, 05.08.2019 00:00



 is 1.15
is 1.15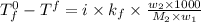
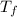 = freezing point of solution =
= freezing point of solution = 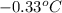
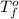 = freezing point of water =
= freezing point of water = 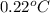
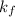 = freezing point constant of water =
= freezing point constant of water = 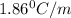
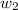 = mass of solute = 1.168 g
= mass of solute = 1.168 g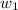 = mass of solvent (water) = 21.797 g
= mass of solvent (water) = 21.797 g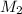 = molar mass of solute = 208 g/mol
= molar mass of solute = 208 g/mol