
Chemistry, 31.03.2020 00:59 charnaetoney13
Na2CO3(aq)+2HCl(aq)→2NaCl(aq)+H2O(l )+CO2(g) A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the solute and the other contained HCl . The chemical reaction that took place is represented by the equation above. What experimental result would be evidence that a chemical reaction took place when the solutions were combined?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Na2CO3(aq)+2HCl(aq)→2NaCl(aq)+H2O(l )+CO2(g) A student combined two colorless aqueous solutions. One...
Questions


Physics, 18.08.2021 16:40

English, 18.08.2021 16:40

Physics, 18.08.2021 16:40

Mathematics, 18.08.2021 16:40

Geography, 18.08.2021 16:40


Mathematics, 18.08.2021 16:40



Arts, 18.08.2021 16:40

English, 18.08.2021 16:40

Biology, 18.08.2021 16:40





Mathematics, 18.08.2021 16:40

Mathematics, 18.08.2021 16:40

Physics, 18.08.2021 16:40

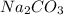 and HCl chemically combine together leading to the formation of NaCl, water and carbon dioxide gas. As the gas is forming and its formation will also form bubbles into the solution.
and HCl chemically combine together leading to the formation of NaCl, water and carbon dioxide gas. As the gas is forming and its formation will also form bubbles into the solution.

