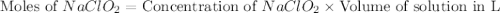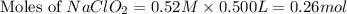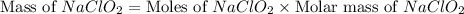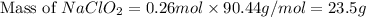Chemistry, 31.03.2020 01:01 nestergurl101
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid is a weak acid with =Ka×1.110−2. What mass of NaClO2 should the student dissolve in the HClO2 solution to turn it into a buffer with pH =2.11?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
You know the right answer?
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid...
Questions

Mathematics, 02.11.2020 02:30

Mathematics, 02.11.2020 02:30







History, 02.11.2020 02:30

Mathematics, 02.11.2020 02:30





Mathematics, 02.11.2020 02:30

Mathematics, 02.11.2020 02:30

Mathematics, 02.11.2020 02:30


Health, 02.11.2020 02:30

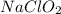 should be, 23.5 grams.
should be, 23.5 grams.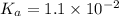
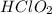 = 0.40 M
= 0.40 M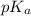 .
.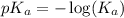
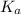 in this expression, we get:
in this expression, we get: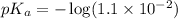
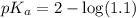
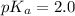
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0572/2296/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0572/2296/a8df0.png)
![2.11=2.0+\log (\frac{[NaClO_2]}{0.40})](/tpl/images/0572/2296/3a0b0.png)
![[NaClO_2]=0.52M](/tpl/images/0572/2296/2adfa.png)