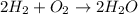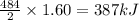Chemistry, 31.03.2020 01:21 LtotheJ0225
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the transfer of heat that occurs when 1.60 mol H 2 reacts? 387 kJ absorbed 484 kJ released 387 kJ released 774 kJ absorbed 484 kJ absorbed 774 kJ released

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the trans...
Questions

Mathematics, 26.06.2021 18:30


Chemistry, 26.06.2021 18:30


Chemistry, 26.06.2021 18:30

Physics, 26.06.2021 18:30

Mathematics, 26.06.2021 18:30

Computers and Technology, 26.06.2021 18:30


History, 26.06.2021 18:30

History, 26.06.2021 18:30

Mathematics, 26.06.2021 18:30


English, 26.06.2021 18:30


Health, 26.06.2021 18:30

Mathematics, 26.06.2021 18:30

Mathematics, 26.06.2021 18:30

SAT, 26.06.2021 18:30

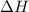 is positive.
is positive.