

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Chlorine atoms react with methane, forming HCl and CH3. The rate constant for the reaction was deter...
Questions

English, 21.12.2020 01:10






History, 21.12.2020 01:10

Mathematics, 21.12.2020 01:10




Mathematics, 21.12.2020 01:10

Chemistry, 21.12.2020 01:10




Computers and Technology, 21.12.2020 01:10

Mathematics, 21.12.2020 01:10


![\ln(\frac{K_{317K}}{K_{278K}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0572/2836/530df.png)
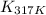 = equilibrium constant at 317 K =
= equilibrium constant at 317 K = 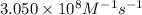
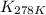 = equilibrium constant at 278 K =
= equilibrium constant at 278 K = 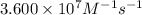
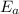 = Activation energy = ?
= Activation energy = ?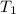 = initial temperature = 278 K
= initial temperature = 278 K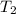 = final temperature = 317 K
= final temperature = 317 K![\ln(\frac{3.050\times 10^8}{3.600\times 10^{7}})=\frac{E_a}{8.314J/mol.K}[\frac{1}{278}-\frac{1}{317}]\\\\E_a=40143.3J/mol=40.143kJ/mol](/tpl/images/0572/2836/701bc.png)



