Chemistry, 31.03.2020 01:28 rainbow7020
A sample of 8.4 grams of NaOH is dissolved into 620 mL of aqueous 0.250 M NaOH (assume no volume change). This solution is then poured into 1.65 gallons of water. (You may assume that the two volumes can be added.) What is the concentration of NaOH in the final solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
A sample of 8.4 grams of NaOH is dissolved into 620 mL of aqueous 0.250 M NaOH (assume no volume cha...
Questions

Chemistry, 21.04.2021 04:40



Physics, 21.04.2021 04:40

History, 21.04.2021 04:40

English, 21.04.2021 04:40

Health, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40



Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

English, 21.04.2021 04:40


Mathematics, 21.04.2021 04:40


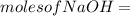
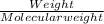
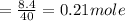 620 ml of aqueous 0.25 M NaOH
620 ml of aqueous 0.25 M NaOH 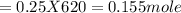 Total moles = 0.21 + 0.155 = 0.365 moles1.65 gallons = 3.785 lit x 1.65 = 6.245929 lit (∵ 1 gallon = 3.785 lit)
Total moles = 0.21 + 0.155 = 0.365 moles1.65 gallons = 3.785 lit x 1.65 = 6.245929 lit (∵ 1 gallon = 3.785 lit)