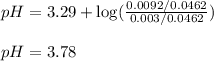Chemistry, 31.03.2020 01:36 abbypoletick
When a 26.3 mL sample of a 0.465 M aqueous nitrous acid solution is titrated with a 0.461 M aqueous barium hydroxide solution, what is the pH after 19.9 mL of barium hydroxide have been added

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
When a 26.3 mL sample of a 0.465 M aqueous nitrous acid solution is titrated with a 0.461 M aqueous...
Questions


Mathematics, 21.05.2021 22:00



History, 21.05.2021 22:00

History, 21.05.2021 22:00

Mathematics, 21.05.2021 22:00





Mathematics, 21.05.2021 22:00



Mathematics, 21.05.2021 22:00

English, 21.05.2021 22:00

Mathematics, 21.05.2021 22:00

Mathematics, 21.05.2021 22:00


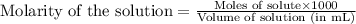 .......(1)
.......(1)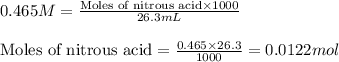
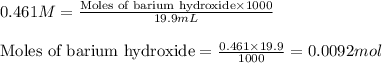
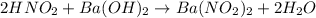
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0572/3345/e4eea.png)
![pH=pK_a+\log(\frac{[NO_2^-]}{[HNO_2]}](/tpl/images/0572/3345/c621f.png)
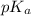 = negative logarithm of acid dissociation constant of nitrous acid = 3.29
= negative logarithm of acid dissociation constant of nitrous acid = 3.29![[NO_2^-]=\frac{0.0092}{0.0462}](/tpl/images/0572/3345/4843c.png)
![[HNO_2]=\frac{0.003}{0.0462}](/tpl/images/0572/3345/5be58.png)