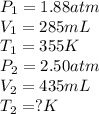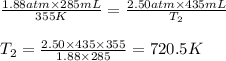Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
A syringe initially holds a sample of gas with a volume of 285 mL at 355 K and 1.88 atm. To what tem...
Questions


Mathematics, 14.10.2019 14:10


Biology, 14.10.2019 14:10

Biology, 14.10.2019 14:10


Mathematics, 14.10.2019 14:10

Health, 14.10.2019 14:10

History, 14.10.2019 14:10


Health, 14.10.2019 14:10



History, 14.10.2019 14:10



Mathematics, 14.10.2019 14:10

Mathematics, 14.10.2019 14:10


Chemistry, 14.10.2019 14:10

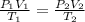
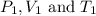 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas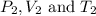 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas