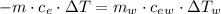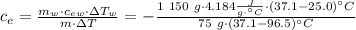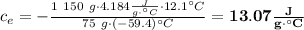Chemistry, 22.09.2019 00:30 Arianahinton9856
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the final temperature of the system is 37.1°c, what is the specific heat capacity of the substance? use 4.184 j / g °c for the specific heat capacity of water.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0 c?
Answers: 1
You know the right answer?
Achemist mixes 75.0 g of an unknown substance at 96.5°c with 1,150 g of water at 25.0°c. if the fina...
Questions




History, 20.04.2021 18:10

Biology, 20.04.2021 18:10


Computers and Technology, 20.04.2021 18:10


Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

English, 20.04.2021 18:10


History, 20.04.2021 18:10

History, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Chemistry, 20.04.2021 18:10


Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10