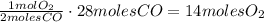Chemistry, 31.03.2020 02:59 camiserjai1832
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emissions such as carbon monoxide, CO(g), into carbon dioxide, CO2(g). The uncatalyzed reaction is represented by the balanced equation below.2CO(g) O2(g) 2CO2(g) heatDetermine the number of moles of O2(g) required to completely react with 28 moles of CO(g) during this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2


Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emission...
Questions





Social Studies, 11.11.2020 19:40


Mathematics, 11.11.2020 19:40

History, 11.11.2020 19:40

English, 11.11.2020 19:40



Mathematics, 11.11.2020 19:40





Social Studies, 11.11.2020 19:40

Health, 11.11.2020 19:40


Mathematics, 11.11.2020 19:40