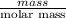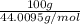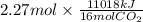Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the fo...
Questions

Mathematics, 08.04.2021 02:50

Mathematics, 08.04.2021 02:50


Chemistry, 08.04.2021 02:50



English, 08.04.2021 02:50


Mathematics, 08.04.2021 02:50

Mathematics, 08.04.2021 02:50


Mathematics, 08.04.2021 02:50


Mathematics, 08.04.2021 02:50



Mathematics, 08.04.2021 02:50


Mathematics, 08.04.2021 02:50

Mathematics, 08.04.2021 02:50

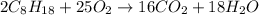 ;
; 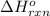 = -11018 kJ
= -11018 kJ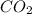 = 100 g
= 100 g